La distribución en la población de lo que llamamos alta miopía o miopía extrema es muy difícil de cuantificar, pues no existe una delimitación absolutamente precisa entre las diferentes magnitudes.
Las delimitaciones no son claras a la hora de clasificarlas, ni existe consenso si debemos diferenciarlas por el largo axial o por la refracción, o por el poder del lente intraocular a implantar. Tampoco se han dilucidado los umbrales que dividen las distintas categorías.
En este sentido se puede observar que, arbitrariamente, en algunos trabajos, se dividen solamente entre miopía leve, hasta –3 D por un lado, y por el otro, se ubica la moderada junto con la elevada, por encima de –3 D. Otros estudios hablan de elevada a partir de –8 D y extrema por encima de –10 D 2.
El ojo miope extremo, particularmente, constituye un verdadero desafío para el cirujano de segmento anterior por varios motivos, que analizaremos a continuación.
Estos pacientes nos ofrecen una problemática muy peculiar, que debe ser considerada como tal, analizada en toda su complejidad y enfrentada en cada caso como una situación individual y no como si se tratara de una catarata en un ojo de dimensiones normales.
Pueden aparecer escollos en los 2000, es un método con un error de todos los pasos del proceso: durante el estudio preoperatorio, en la etapa intraoperatoria y también en el posoperatorio. En cada momento es necesario advertir sobre los posibles inconvenientes a los que nos enfrentaremos, evitar minimizarlos, para no transformarlos en un caso de difícil manejo.
Los avances tecnológicos en la evaluación preoperatoria, el cálculo del LIO mucho más exacto, la incorporación de lentes intraoculares multifocales, trifocales y de rango extendido han transformado la cirugía sobre el cristalino en una cirugía refractiva. Es en este contexto en el que la cirugía de catarata en el ojo miope extremo debe ser analizada a la hora de realizar el implante del lente intraocular.
Evaluación preoperatoria:
Como en todo paciente que se somete a una cirugía de catarata, debe realizarse un examen oftalmológico completo, pesquisando primero si existen trastornos de la superficie ocular, y tratándolos con firmeza antes de realizar la biometría.
El cálculo del lente es fundamental a la hora de implantar cualquier lente intraocular, pero en estos casos es capital. La biometría ultrasónica, única utilizada hasta principios de medida inaceptable para los requerimientos actuales. Esos errores de medida del largo axial pueden llegar a ser tan grandes, en los casos de los ojos extremos, que las variaciones queratométricas debido a la superficie ocular pueden tener poca influencia en el resultado final. En la era actual del cálculo, con la biometría óptica, los errores que generan córneas irregulares pueden ser muy significativos debido a que el error de cálculo tolerado es mínimo.
Los ojos con largos axiales mayores que 30 mm son los que más frecuentemente consideramos cuando nos referimos a ojos miopes extremos.
Estos largos muy extremos pueden combinarse con córneas normales, pero también con córneas muy planas o con córneas muy curvas. Sin embargo, debemos tener en cuenta que, largos no tan extremos, entre 26 y 30 mm, pueden combinarse con córneas muy curvas y resultar en la necesidad de implantar lentes intraoculares en un rango entre –10 D y +3 D.
Cada una de estas combinaciones puede generar un error diferente a la hora del cálculo del lente intraocular.
A estos tipos de ojo miope extremo se deben sumar algunos pocos pacientes que fueron operados en la época inicial de la cirugía con excimer, cuando se operaban miopías de –15 D, que son muy difíciles de calcular, debido a que tienen áreas tratadas con diámetro muy pequeño, extremadamente planas en el centro, y que nos generan problemas por lo exagerado del aplanamiento corneal y la dificultad en las mediciones queratométricas por las irregularidades topográficas que acompañan a este tipo de ablación.
También debemos considerar los miopes extremos operados previamente con lentes intraoculares fáquicos, de cámara anterior con soporte angular, tipo Kelman, o de soporte iridiano de tipo iris claw, o los de cámara posterior. En estos casos, se agrega la dificultad quirúrgica que significa la extracción del lente fáquico y el astigmatismo que puede generar la incisión dependiendo del modelo que deba ser explantado: de 6 o 6.5 mm en los que son de PMMA (cámara anterior de soporte angular e iridiano) y de 3.25 o 3.5 mm en los plegables (Artiflex, ICL), que son de polisiloxano y Collamer, respectivamente. El cálculo en estos ojos requiere corregir el largo axial en el IOLMaster o Lenstar con la opción de ojo con lente fáquico, agregando el tipo de material del lente utilizado y calcularlo como un ojo miope elevado, como si no estuviera operado.
La introducción de la Interferometría de Coherencia Parcial en 1998 ha mejorado la medida del largo axial dramáticamente. En la actualidad es imprescindible efectuarla siempre en la miopía extrema.
Ante la imposibilidad de realizarla, por ejemplo, en cataratas completas, subcapsulares posteriores u otras circunstancias con opacidad de medios muy importante, deberemos hacer la biometría ultrasónica solamente cuando no se pueda hacer la biometría óptica.
Con la biometría ultrasónica sabemos que estamos cometiendo un error de medida que puede ser muy importante, y que, aunque tomemos todos los recaudos en la medición y en el resto de los parámetros, es altamente probable que el cálculo del lente a implantar nos conduzca a un error refractivo frecuentemente significativo.
La principal ventaja de la biometría óptica es su mayor precisión, 10 veces, comparada con la biometría ultrasónica. Ha mejorado el cálculo en general, utilizando cualquiera de las fórmulas disponibles.
La utilización de los MIOL nos obliga a obtener resultados refractivos entre +0.50 D y –0.50 D idealmente en todos los casos. Esta exactitud no la podemos prometer en los miopes extremos todavía, aunque ha habido avances prometedores en el cálculo en los últimos años, ese nivel de exactitud no puede ser alcanzado en este tipo de ojos.
La evaluación de la retina periférica con oftalmoscopía binocular indirecta debe ser realizada siempre para detectar desgarros o lesiones retinales que predispongan al desprendimiento de retina. Además, se debe evaluar la mácula con tomografía de coherencia óptica (OCT), para detectar alteraciones maculares, que se debe realizar como en todos los casos, aunque en los miopes elevados no siempre se puede obtener una buena imagen macular en el OCT.
Chair Time
• En el interrogatorio, el primer tema a considerar es analizar con el paciente cuáles son sus expectativas y cuáles son sus posibilidades reales. En este contexto, es frecuente que los pacientes estén informados sobre los tipos de lentes intraoculares disponibles en el mercado, pero no saben que no todos son aplicables a su caso en particular. En este sentido, es importante anticiparle que, luego de los estudios, veremos cuál o cuáles se adaptan mejor a las características de su ojo.
• Esto incluye el tema de la corrección de astigmatismos corneales, que se debe tener en cuenta, pero creo que es inútil conversar, previamente, de alternativas muy especiales sin sustento y después, frente a los resultados, comenzar a explicar por qué en su caso no puede implantarse un tipo u otro de lente de los que ya hemos hablado anteriormente de manera un tanto superficial.
• Una pregunta que no puede faltar debe ir orientada a saber cómo se ha manejado habitualmente durante la vida. (En los que tienen solo catarata monocular hay que plantear el tema en función de ambos ojos)
◊ Aquellos que han usado anteojos aéreos solamente, deben comprender que, una vez operados, pueden llegar a ver muy bien de lejos, pero eso implicaría ver muy mal de cerca. En este sentido, si prefieren ver
de lejos sin anteojos, usarán anteojos de cerca. Otra alternativa, en los pacientes que prefieren seguir manteniendo buena visión cercana sin corrección y no les molestaría usar anteojos aéreos para la visión lejana, con una corrección de alrededor de–3D.
◊ Por otro lado, están los pacientes con miopía extrema, que vienen utilizando solamente lentes de contacto y no utilizaron nunca corrección de cerca. En este punto es necesario tratar de preguntar si han utilizado en monovisión, aunque puede ser que hayan estado hipocorregidos ambos, y con diferencia entre ambos ojos. Estos pacientes se beneficiarán seguramente con un resultado refractivo de –1.00 D a –2.50 D.
◊ Aquellos que previamente utilizaron lentes de contacto para ver bien a distancia, pero usaron siempre corrección de cerca, y eso es lo que prefieren, es mejor apuntar a la emetropía o a una miopía de –0.50 D.
◊ También es muy diferente en los casos operados con lentes fáquicos, en los cuales es fundamental saber cómo han estado previamente, de la misma manera que con los usuarios de LC.
◊ Si se trata de pacientes pre présbitas que han utilizado siempre LC y nunca anteojos, la situación exige una conversación mayor que consiste en explicar la presbicia a alguien que no la ha padecido todavía y que está acostumbrado a ver bien de lejos y cerca con LC. Existen muchos pacientes jóvenes con cataratas en miopías elevadas.
• Desde el punto de vista del compromiso de la retina, es importante tener en cuenta que el riesgo del desprendimiento de retina es mayor en este grupo de pacientes. Hay que advertirlo, anticiparlo, como explicamos el riesgo de endoftalmitis en lo relativo a la frecuencia con la que puede aparecer y sus consecuencias.
En este sentido, se ha propuesto clasificar la miopía elevada en: aislada, que no presenta patología retinal, y ocurre en aproximadamente el 50 % (IHM); patológica anterior, el 22 % (APM), cuando está afectada la retina anterior; patológica posterior, el 10 % (PPM), cuando está afectada la mácula; y combinada, el 17 % (CPM), que incluye anterior y posterior. El equivalente esférico promedio de cada grupo aumenta desde la aislada (–8 D) a la combinada (–10 D)1. Este estudio nos indica que, de cada dos miopes extremos, uno puede tener lesión patológica retinal, y el otro puede no tenerla.
Evaluación preoperatoria
• Sedebecitarpararealizarlosestudios preoperatorios con tiempo suficiente de descanso de los LC. El examen debe incluir biometría de coherencia óptica y topografía corneal con aberrometría. En este sentido, existen diversos equipos como Pentacam, Galilei, OPD Sense III, que aportan muchos datos sobre la córnea, la superficie ocular y el sistema óptico que hoy constituyen herramientas indispensables para la selección del lente intraocular.
• Como dije anteriormente, un tema en absoluto no menor, es que la gran mayoría de los pacientes miopes extremos son usuarios habituales de lentes de contacto y muy reacios a suspenderlos por tiempos prolongados. Esto genera dos problemas: las mediciones corneales pueden ser de muy mala calidad, debido a la alteración de la superficie ocular por el sobreuso, y el fenómeno de moldeado de la córnea (síndrome de warpage), que puede dar lugar a errores en las medidas queratométricas. En general se aconseja discontinuar los lentes por lo menos una a dos semanas en el caso de los blandos y 4 a 6 semanas en los rígidos.
• Uno de los factores que conspira contra el éxito en la exactitud de la medida del largo axial es que, si bien la precisión de la biometría basada en la interferometría de coherencia óptica ha mejorado mucho, la ubicación geográfica de la fóvea en estos ojos no siempre es posible, por lo que no se puede medir correctamente el largo axial.
◊ En el caso de los ojos miopes extremos, en primer lugar, se trata de un ojo con diversas anomalías en cuantoasuformaynosolo a sus dimensiones. Estas anomalías del perfil posterior retinal, como los estafilomas, modifican la superficie cóncava regular y generan otra irregular, muy particularmente inclinada, que no mantiene un radio de curvatura posterior preestablecido, y crean un punto foveal ubicado en una posición que noesladeunojocon dimensiones medias. La ecografía modo B ayuda a visualizar y valorar la morfología de esta deformación posterior, que puede crear un desnivel de unos 2 mm, pero no es útil para medir.
◊ En el momento de la medición del largo axial, algunos miopes muy elevados no llegan a fijar muy bien, es decir, pierden nitidez al mirar la luz de fijación, debido a la enorme ametropía. En este caso, un tip que puede ayudar a perfeccionar la medida del largo axial con el IOLMaster es utilizando la refracción aérea y realizar varias tomas para corroborar con las anteriores que hemos obtenido sin ella.
Elección del poder del lente intraocular
• Otradificultadquesesuma en esos rangos de lentes intraoculares es que hay pocos modelos que abarquen todo el abanico de poderes. Algunos modelos de lentes intraoculares comienzan en +6 D, otros en 0 D, otros en –6 D y otros en –15 D. La diferencia entre los poderes en este rango es de 1 D, no de 0.50 D, como en los restantes lentes intraoculares, desde +6 D hasta +30 D. Esta diferencia de +1 D crea algunos errores que se originan a la hora de elegir el poder. Por ejemplo: si el cálculo nos ofrece un resultado de +2.25 D, debemos colocar un de +3 o +2 D, cuando quizás hubiéramos elegido uno de +2.50 D.
Otro tema frecuente en estos ojos es que encontramos discordancias significativas entre las diversas fórmulas. Las diferencias surgen del método que cada fórmula utiliza para calcular la posición efectiva del lente (ELP). Holladay presentó hace más de 10 años los nueve tipos de ojos según el largo axial y la dimensión del segmento anterior. En el 90 % de los ojos largos encontró que el segmento anterior está en valores de rango normal, y en el 10 % solamente es más grande. Si la ELP se calcula infiriéndola, solo teniendo en cuenta el largo axial, el resultado será muy diferente en los ojos con segmento anterior normal o aquellos que en los que es más largo. Este error no se produce si es que existe un dato real de la medida donde se cree que va a quedar la lente intraocular, que se calcula con medidas específicas de la profundidad de la cámara anterior y del espesor del cristalino. Existen en esta franja de ojos numerosos estudios comparando las diversas fórmulas.
◊ En general, entre las fórmulas más frecuentemente usadas hasta ahora, la Haigis y la SRKT funcionan mejor. Haigis tiene menor error que la SRKT en los ojos con K mayor que 43 D y largos axiales mayores que 30 mm3.
◊ Las fórmulas más nuevas de Olsen y Barrett Universal II van ganando terreno últimamente ya que miden fehacientemente el espesor del cristalino y la cámara anterior, calculando más exactamente la posición en la que el LIO va a posicionarse dentro del bag. En otro estudio se analiza el error refractivo en la predicción tomando en cuenta nueve fórmulas4 y dos equipos: Lenstar y IOLMaster. Los resultados demostraron a la Olsen como la más exacta con las mediciones del Lenstar, pero fue la peor con los valores obtenidos por el IOLMaster; la Barrett Universal II fue la mejor con las medidas del IOLMaster. La de Olsen que viene en el equipo (Lenstar) funcionó peor que la versión Standalone Olsen, que se puede acceder a través de la página web PhacoOptics PC.
En otro estudio comparando dos series de fórmulas preinstaladas en Lenstar e IOLMaster, se obtuvo mejor resultado con Olsen, seguido de la Haigis, en todos los tipos de ojo5.
◊ Otro método para calcular el poder del lente en ojos mayores que 25 mm es el del ajuste del largo axial, que proponen Koch y Wang, usando la fórmula Holladay 1 (ALoptimized Holladay I), aumentando +0.27 a +0.68 D en los poderes de lentes intraoculares arriba de +5 D y +1.13 a +1.87 D en los lentes por debajo de 5 D 6.
◊ Personalmente, desde 2012, a partir de un estudio retrospectivo personal, utilizo un método con el que optimizo el cálculo para evitar el shift hipermetrópico: calculo con IOLMaster, fórmula de Haigis optimizada, sumando +0.75 para la emetropía en todos los casos, pero agregando 1 D adicional en ojos con largos axiales mayores que 30 mm, K superiores7 a 44 D o ACD mayor que 3.8 mm .
◊ En 2017 comenzó a utilizarse la fórmula de Warren Hill-RBF que es una fórmula basada en principios de la inteligencia artificial. Se puede acceder a través de https//rbfcalculator.com. El calculador puede medir de 19 a 35 mm de largo
axial. El método consiste en la incorporación a su base de datos de más de 12 000 casos operados, con los resultados posquirúrgicos, donde un modelo de inteligencia artificial encuentra similitudes y sugiere el lente a implantar. La exactitud de este método en algunos trabajos es muy promisoria.
◊ Otro método de cálculo es con la aberrometría intraoperatoria con el ORA, que algunos estudios lo posicionan como el más exacto.
Elección del lente que vamos a implantar
• Una vez calculado el poder de la lente intraocular, y con el resultado de la topografía y la biometría óptica, volvemos a conversar con el paciente sobre las posibilidades refractivas que podemos ofrecerle.
◊ En esta decisión uno debe considerar: que no siempre la emetropía es lo mejor, que la hipermetropía es siempre lo peor y que una leve miopía puede ser el mejor resultado.
◊ En general no propicio la utilización de lentes intraoculares multifocales en la miopía extrema. El multifocal de menor poder que he implantado es de +8 D en un ojo, en una paciente con una anisometropía que llevaba +15 D en el otro y con muy buen resultado. Hasta hace unos años, era una situación impensada, debido a que la mayoría de los lentes intraoculares multifocales comenzaban en +10 D y luego se expandieron a +6 D.
◊ En la actualidad existen algunos modelos desde 0 D, lo cual creo es peligroso, pues todavía es muy difícil garantizar obtener un valor suficientemente exacto del poder esférico del lente que permita una buena función en multifocalidad, debido a un error refractivo posoperatorio inesperado.
◊ También hay que tener en cuenta que muchas veces podemos no disponer un stock de tóricos, con la imposibilidad de corregir el cilindro corneal preoperatorio.
◊ El otro tema, no menor, a la hora de la elección del LIO, es el diámetro total entre las hápticas, pues en ojos extremadamente grandes un LIO estándar monopieza puede quedar inestable dentro de un saco grande. En estos casos, uno de tres piezas, de 13 mm de diámetro, se acomoda mejor en un saco capsular grande, mientras que uno de 12 mm puede desplazarse dentro del saco, dando lugar a descentraciones que pueden ser significativas en los casos de los lentes asféricos.
Como conclusión, hay que ser prudente, muy meticuloso, explicar mucho, advertir sobre riesgos y no crear expectativas descabelladas.
Referencias
1. Ludwig CA, Shields RA, Chen TA. A novel classifica- tion of high myopia. Graefes Arch Clin Exp Ophthal- mol 2018 oct 256(10) 1847-56
2. Cumberland P, Bountziouka V, Rahi J. Impact of varying the definition of myopia on estimates of prevalence and associations with risk factors. Br J Ophthalmol 2018 OCT: 102(102): 1407-12
3. ZhuXJ,HeWW,DuY,QianDJ,DaiJH,LuY.Lens power calculation for high myopic eyes with cataract: comparison of three formulas. Chin J Ophthalmol, 2017, 53: 260-265.
4. Cooke DL, Cooke TL J Comparison of 9 intraocular lens power calculation formulas. JCataract Refract Surg. 2016 Aug;42(8):1157-64
5. Cooke DL, Cooke TL. Prediction accuracy of preinsta- lled formulas on 2 optical biometers. J Cataract Re- fract Surg. 2016 Mar;42(3):358-62.
6. Wang L, Shirayama M, Ma X J, Koch D. Optimizing intraocular lens power calculations in eyes with axial lengths above 25 mm. J Cataract Refract Surg. 2011 Nov 37 (11): 2018-27.
7. Fernandez Mendy J; Fernandez Mendy A; Fernandez Mendy H. Optimizando el Cálculo en Miopes Altos. Presentado en el Congreso Panamericano de Oftal- mología. Lima 2017.





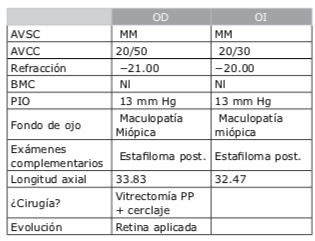
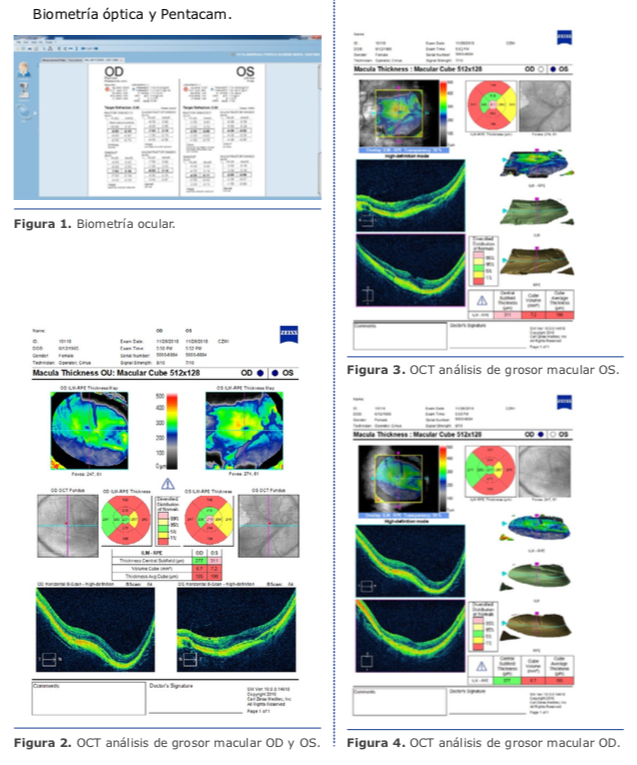
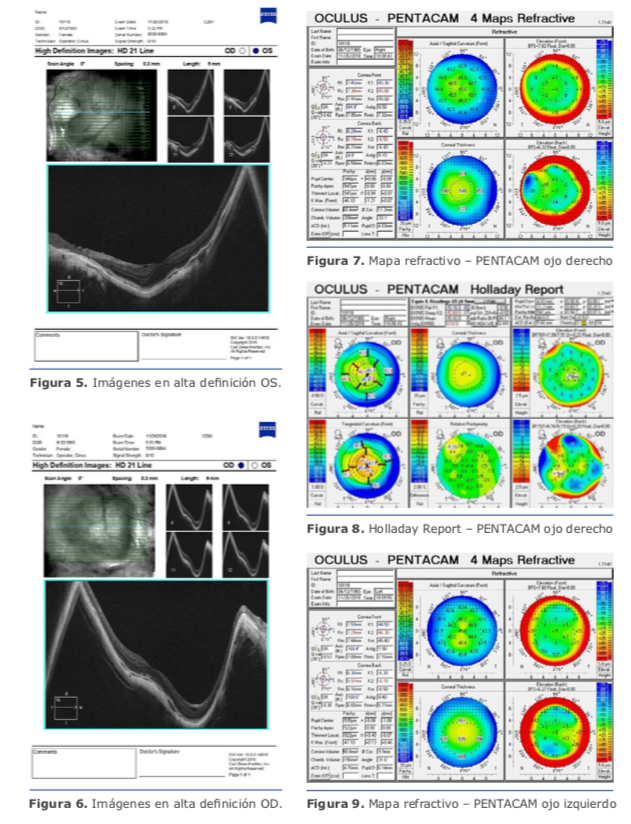
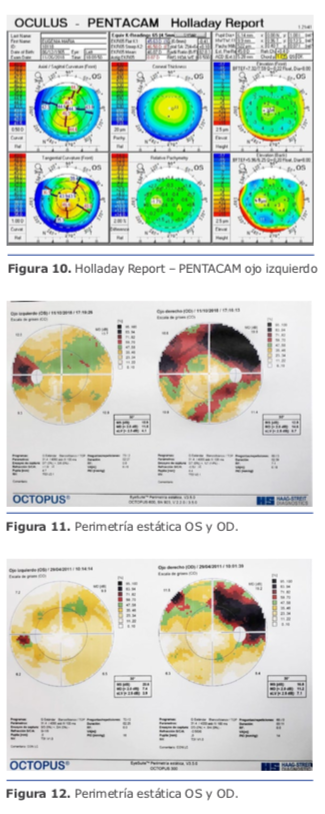 Dr. Pablo Suárez: Para el cálculo de la lente debemos tener varios puntos claros en cuanto a la medición de los diferentes compartimentos del ojo. Para ello, creo que se deben valorar los siguientes tipos de ojos miopes:
Dr. Pablo Suárez: Para el cálculo de la lente debemos tener varios puntos claros en cuanto a la medición de los diferentes compartimentos del ojo. Para ello, creo que se deben valorar los siguientes tipos de ojos miopes: Dr. Juan Guillermo Ortega: Los cálculos de miopías extremas suelen producir hipermetropía posoperatoria porque magnifican el poder del lente a implantar. Este hallazgo produjo lo que se denomina el ajuste de Wang-Koch, que es particularmente útil en fórmulas antiguas como Holladay 1, y que originalmente se proponía para longitudes axiales superiores a 25,2 mm. SRK/T se comporta mejor, y Haigis todavía más, porque es menos sensible a esta aberración de medida óptica. Las fórmulas más recientes como Barrett Universal, Hill RBF y PANACEA se comportan mucho mejor en estos casos. (Ver figura 15)
Dr. Juan Guillermo Ortega: Los cálculos de miopías extremas suelen producir hipermetropía posoperatoria porque magnifican el poder del lente a implantar. Este hallazgo produjo lo que se denomina el ajuste de Wang-Koch, que es particularmente útil en fórmulas antiguas como Holladay 1, y que originalmente se proponía para longitudes axiales superiores a 25,2 mm. SRK/T se comporta mejor, y Haigis todavía más, porque es menos sensible a esta aberración de medida óptica. Las fórmulas más recientes como Barrett Universal, Hill RBF y PANACEA se comportan mucho mejor en estos casos. (Ver figura 15)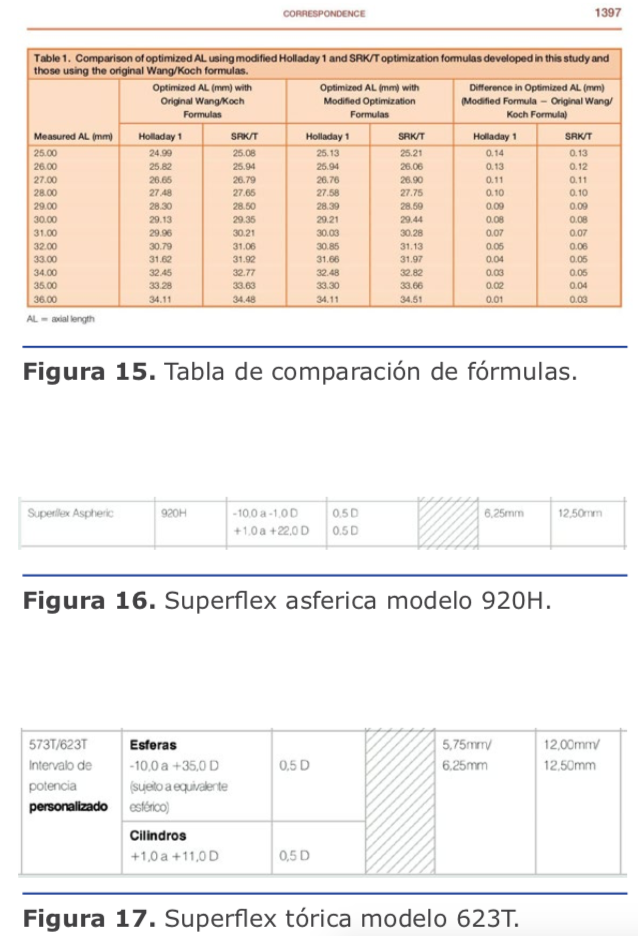 Esta lente es de una sola pieza de acrílico (Rayacril) de borde cuadrado en 360 grados con una baja incidencia de opacidad capsular muy importante para evitar o retrasar lo más posible la apertura de la cápsula posterior con YAG en estos tipos de pacientes, y una óptica grande de 6,25 mm que permite realizar un examen minucioso de la retina periférica para el control o posibles tratamientos a futuro, por 12.50 mm de longitud ideal para estos pacientes que presentan un bag grande. (Ver figura 18)
Esta lente es de una sola pieza de acrílico (Rayacril) de borde cuadrado en 360 grados con una baja incidencia de opacidad capsular muy importante para evitar o retrasar lo más posible la apertura de la cápsula posterior con YAG en estos tipos de pacientes, y una óptica grande de 6,25 mm que permite realizar un examen minucioso de la retina periférica para el control o posibles tratamientos a futuro, por 12.50 mm de longitud ideal para estos pacientes que presentan un bag grande. (Ver figura 18)
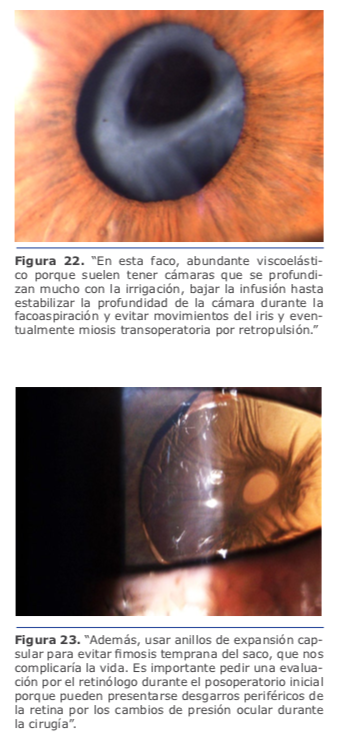 Durante la cirugía trato de evitar cambios bruscos o descompresiones excesivas de la cámara anterior al retirar el faco o la aspiración, siempre colocando viscoelástico o manteniendo la irrigación constante para evitar mayores movimientos del vítreo hacia adelante. En general, realizo un túnel corneal un poco más corto y utilizo una pinza de rexis recta, no curva, para mejor acceso a la cápsula posterior y no abrir el labio de la córnea ni que se deforme el túnel, ya que tenemos que trabajar mucho más abajo por la amplia cámara anterior que tienen este tipo de pacientes. Luego el resto de la cirugía sigue de forma habitual. (Ver figura 21)
Durante la cirugía trato de evitar cambios bruscos o descompresiones excesivas de la cámara anterior al retirar el faco o la aspiración, siempre colocando viscoelástico o manteniendo la irrigación constante para evitar mayores movimientos del vítreo hacia adelante. En general, realizo un túnel corneal un poco más corto y utilizo una pinza de rexis recta, no curva, para mejor acceso a la cápsula posterior y no abrir el labio de la córnea ni que se deforme el túnel, ya que tenemos que trabajar mucho más abajo por la amplia cámara anterior que tienen este tipo de pacientes. Luego el resto de la cirugía sigue de forma habitual. (Ver figura 21)

 Además del dispositivo de ultrasonido digital Insight 100 VHF (ArcScan Inc, Golden, CO), ahora también puede medirse el espesor epitelial mediante sistemas de OCT, especialmente el RTVue − Avanti (Opto-vue, Fremont, CA) y el MS-39. Es de amplio conocimiento que en el queratocono ocurren cambios del espesor epitelial, puesto que curvaturas extremas conducen a la ruptura del epitelio, como se observa a menudo del punto de visto clínico. El adelgazamiento epitelial sobre el cono se ha demostrado mediante análisis histopatológico de córneas con queratocono (Scroggs et al.33) y, posteriormente (Haque et al.34), mediante software personalizado y un sistema de OCT HumphreyZeiss (Humphrey Systems, Dublín, California).
Además del dispositivo de ultrasonido digital Insight 100 VHF (ArcScan Inc, Golden, CO), ahora también puede medirse el espesor epitelial mediante sistemas de OCT, especialmente el RTVue − Avanti (Opto-vue, Fremont, CA) y el MS-39. Es de amplio conocimiento que en el queratocono ocurren cambios del espesor epitelial, puesto que curvaturas extremas conducen a la ruptura del epitelio, como se observa a menudo del punto de visto clínico. El adelgazamiento epitelial sobre el cono se ha demostrado mediante análisis histopatológico de córneas con queratocono (Scroggs et al.33) y, posteriormente (Haque et al.34), mediante software personalizado y un sistema de OCT HumphreyZeiss (Humphrey Systems, Dublín, California).


